Butane
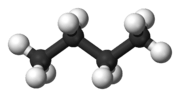
Butane (/ˈbjuːteɪn/) or n-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name butane comes from the roots but- (from butyric acid, named after the Greek word for butter) and -ane. It was discovered by the chemist Edward Frankland in 1849.[6] It was found dissolved in crude petroleum in 1864 by Edmund Ronalds, who was the first to describe its properties.[7][8]
When oxygen is plentiful, butane burns to form carbon dioxide and water vapor; when oxygen is limited, carbon (soot) or carbon monoxide may also be formed. Butane is denser than air.
When there is sufficient oxygen:
When oxygen is limited:
By weight, butane contains about 49.5 MJ/kg (13.8 kWh/kg; 22.5 MJ/lb) or by liquid volume 29.7 megajoules per liter (8.3 kWh/l; 112 MJ/U.S. gal; 107,000 Btu/U.S. gal).
The maximum adiabatic flame temperature of butane with air is 2,243 K (1,970 °C; 3,578 °F).
n-Butane is the feedstock for DuPont’s catalytic process for the preparation of maleic anhydride
